GENERIC VS BRANDED MEDICINES: A Comparison Between Regulated And Non Regulated Markets
What according to you is a healthy nation? Most people would certainly admit that a healthy nation is one where people have access to proper healthcare, and affordable, yet, effective medicines for all. Well, although that would be a perfect and ideal scenario, we are still miles away from achieving

India: Pharmaceutical Legal & Regulatory Environment - Food and Drug Law Institute (FDLI)

Pharmaceutical regulatory requirements of nonregulated markets - ScienceDirect

Comparing Generic Drug Markets in Europe and the United States: Prices, Volumes, and Spending - WOUTERS - 2017 - The Milbank Quarterly - Wiley Online Library

Brand drug vs generic drug
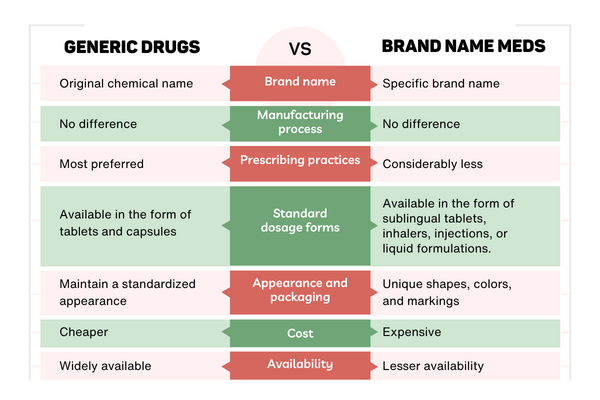
Generic vs. Brand-Name Medications : Choose the better
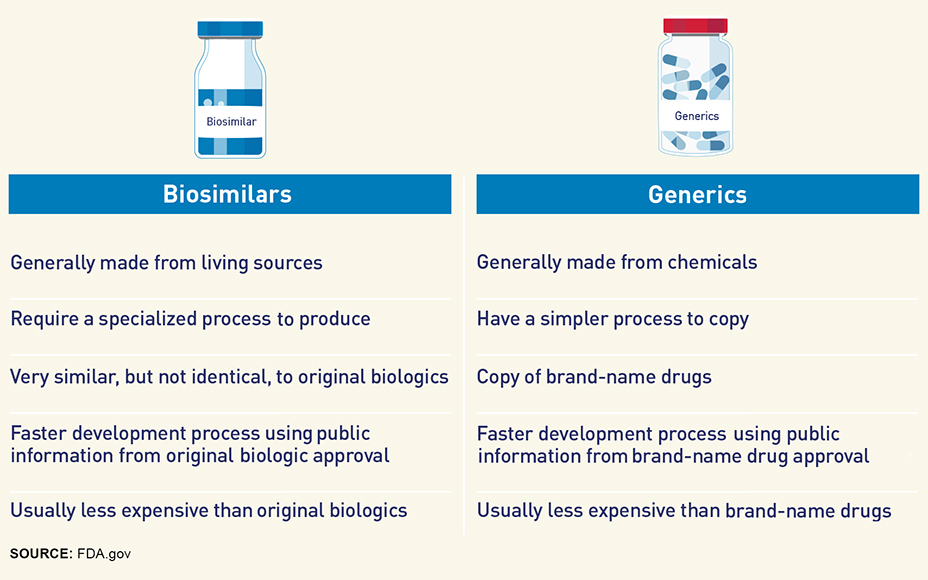
Biosimilars vs. Generics: What's the Difference? - MedBen

Prices, Competition and Regulation in Pharmaceuticals: A Cross-National Comparison - OHE

PDF) REGULATORY REQUIREMENTS FOR REGISTRATION OF GENERIC DRUGS IN “BRICS” COUNTRIES

Differences in Branded and Generic Medicines

PDF) Regulatory Requirements and Registration Procedure for Generic Drugs in USA

Generic vs. Brand-Name Drugs: Is There a Difference?