Tetrahydrobiopterin Prevents Nitration of Tyrosine Hydroxylase by Peroxynitrite and Nitrogen Dioxide

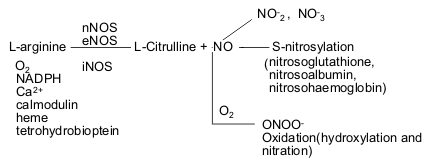
Tyrosine hydroxylase (TH) is the initial and rate-limiting enzyme in the synthesis of the neurotransmitter dopamine. TH is inhibited and nitrated at tyrosine residues in vitro by the reactive nitrogen species peroxynitrite and nitrogen dioxide (NO2) and in vivo by drugs that damage dopamine neurons. Tetrahydrobiopterin, which is the essential cofactor for TH and is concentrated in dopamine neurons, completely blocks nitration of tyrosine residues in TH caused by peroxynitrite or NO2. Various tetrahydro- and dihydro-analogs of tetrahydrobiopterin, including 6,7-dimethyl-tetrahydropterin, 6-methyl-tetrahydropterin, 6-hydroxymethyl-tetrahydropterin, tetrahydropterin, 7,8-dihydrobiopterin, 7,8-dihydroxanthopterin, and sepiapterin, also prevent nitration of tyrosines caused by the reactive nitrogen species. Biopterin and pterin, the fully oxidized forms of the pterin molecule, fail to block peroxynitrite- or NO2-induced nitration of TH. Reduced pterins prevent neither the inhibition of TH activity nor cysteine modification caused by peroxynitrite or NO2, despite blocking tyrosine nitration. However, dithiothreitol prevents and reverses these effects on TH of tetrahydrobiopterin and reactive nitrogen species. Using an enhanced green fluorescent protein-TH fusion construct as a real-time reporter of intracellular tyrosine nitration, tetrahydrobiopterin was found to prevent NO2-induced tyrosine nitration in intact cells but to leave TH activity inhibited. These results indicate that tetrahydrobiopterin prevents the tyrosine-nitrating properties of peroxynitrite and NO2. Tetrahydrobiopterin-derived radical species formed by reaction with reactive nitrogen species may account for inhibition of TH via mechanisms that do not involve tyrosine nitration.

Tetrahydrobiopterin Prevents Nitration of Tyrosine Hydroxylase by Peroxynitrite and Nitrogen Dioxide
Peroxynitrite Biology

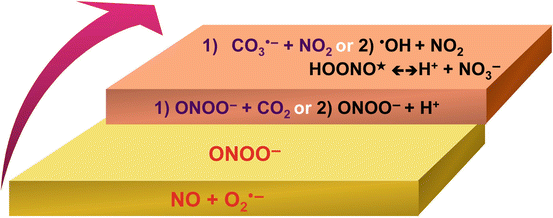
The superoxide radical switch in the biology of nitric oxide and peroxynitrite

Peroxynitrite Inactivation of Tyrosine Hydroxylase: Mediation by Sulfhydryl Oxidation, not Tyrosine Nitration

Biochemistry of Peroxynitrite and Protein Tyrosine Nitration

Nitric Oxide and Peroxynitrite in Health and Disease

Peroxynitrite Biology

Nitric oxide and peroxynitrite in health and disease. - Abstract - Europe PMC

Molecules, Free Full-Text

Drugs modulating the biological effects of peroxynitrite and related nitrogen species - Olmos - 2007 - Medicinal Research Reviews - Wiley Online Library

PDF) Inactivation of Tyrosine Hydroxylase by Nitration Following Exposure to Peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)