BNT162b2 Vaccine Booster and Mortality Due to Covid-19


Effectiveness of mRNA-1273, BNT162b2, and BBIBP-CorV vaccines against infection and mortality in children in Argentina, during predominance of delta and omicron covid-19 variants: test negative, case-control study

Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors

Effectiveness of BNT162b2 and mRNA-1273 COVID-19 boosters against SARS-CoV-2 Omicron (B.1.1.529) infection in Qatar

Effectiveness of a third dose of BNT162b2 mRNA COVID-19 vaccine in a large US health system: A retrospective cohort study - The Lancet Regional Health – Americas
4th dose of mRNA Covid-19 vaccine significantly increases protection in age 60+

Four COVID vaccine doses superior to three in patients with autoimmune rheumatic disease

Effectiveness of homologous and heterologous booster doses for an inactivated SARS-CoV-2 vaccine: a large-scale prospective cohort study - The Lancet Global Health
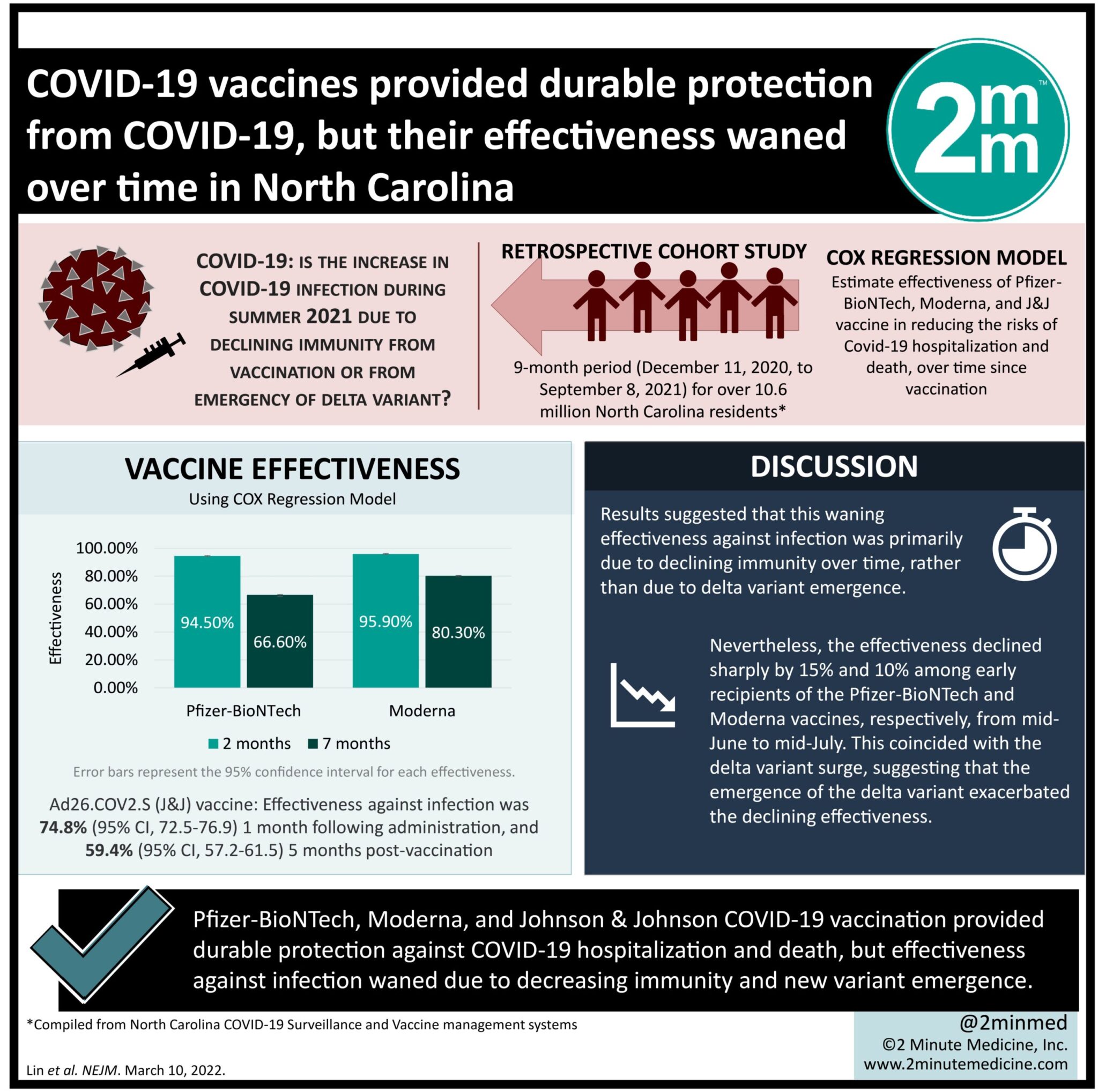
VisualAbstract: COVID-19 vaccines provided durable protection from COVID-19, but their effectiveness waned over time in North Carolina

Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years

mRNA-1273 and BNT162b2 COVID-19 vaccines elicit antibodies with differences in Fc-mediated effector functions

Full article: Effectiveness of BNT162b2 and CoronaVac vaccinations against mortality and severe complications after SARS-CoV-2 Omicron BA.2 infection: a case–control study









