a)Adsorption energy comparison between twice of * NO and N-N

Download scientific diagram | (a)Adsorption energy comparison between twice of * NO and N-N coupling intermediate * ONNO. The horizontal line shows the equilibrium between 2NO(g) and 2 * NO, while the diagonal line illustrates the equal adsorption energy between 2 * NO and * ONNO. (b)The activation barriers for N-N coupling between * N and NO (gas) vs * N and * N. where the unfilled markers show around 0.44 Monolayer of NO coverage is considered. (c) Adsorption energy comparison between * NO and * N 2 O.The horizontal line shows the equilibrium between NO(g) and * NO while the vertical line depicts the equilibrium between N 2 O and * N 2 O under standard conditions. from publication: Electrochemical Nitric Oxide Reduction on Metal Surfaces | Electrocatalytic denitrification is a promising technology for removing NOx species (NO3− , NO2− and NO). For NOx electroreduction (NOxRR), there is a desire for understanding the catalytic parameters that control the product distribution. Here, we elucidate selectivity and | Nitric Oxide, Electrochemistry and CO2 Reduction | ResearchGate, the professional network for scientists.
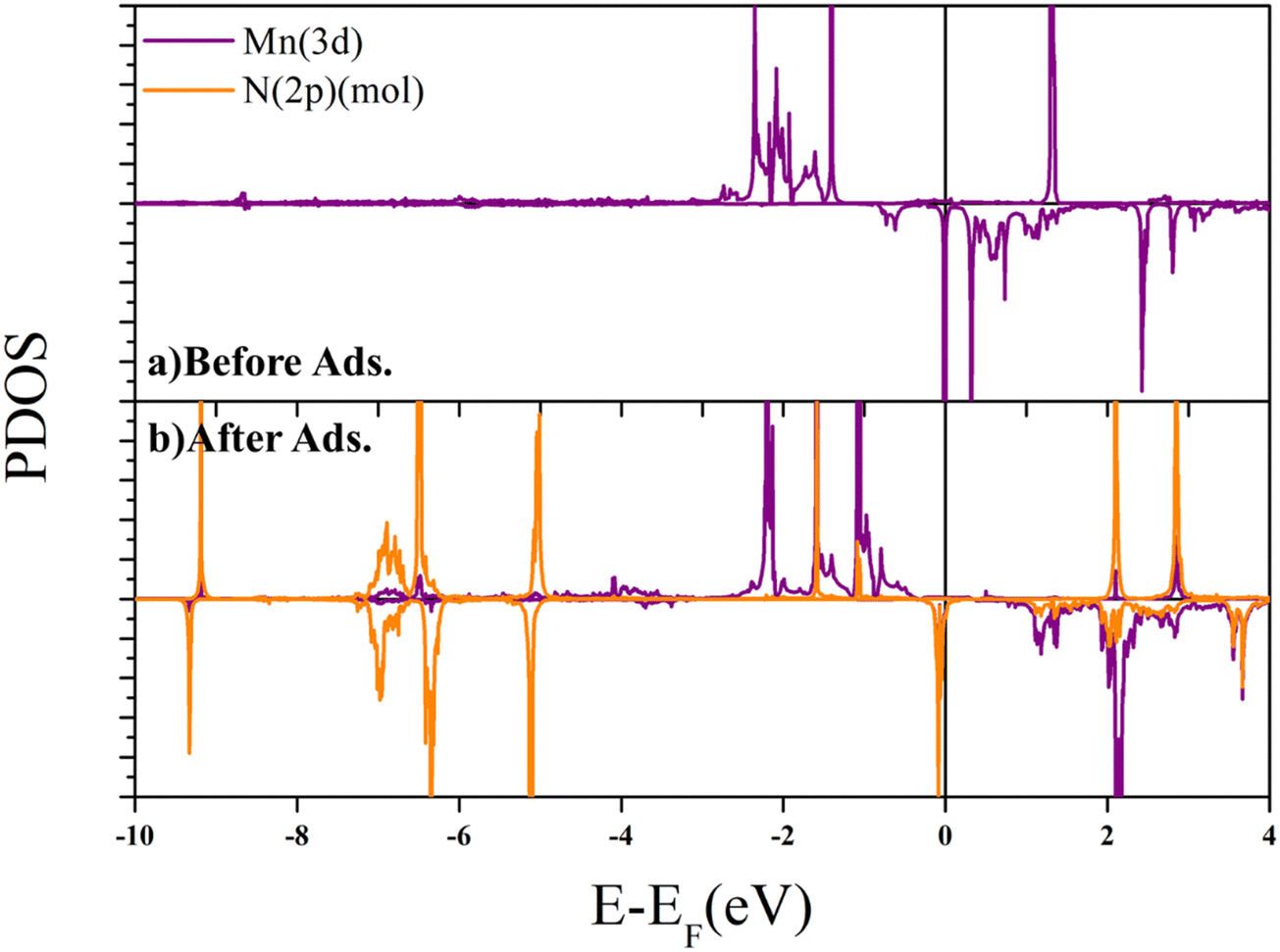
Adsorption mechanism of the N 2 and NRR intermediates on oxygen modified MnN 4 –graphene layers – a single atom catalysis perspective - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/D2CP05491D

DFT calculations for the adsorption states of NO 2 molecules with 2D

The effect of Na/K on the NO adsorption behavior and heterogeneous reduction of internal nitrogen-containing char: A DFT study - ScienceDirect

Density functional theory study of the adsorption of NO on CunX (n = 2–8; X = Cu, K) clusters - Cheng - 2024 - International Journal of Quantum Chemistry - Wiley Online Library

Adsorption Energy - an overview

Determining the adsorption energies of small molecules with the intrinsic properties of adsorbates and substrates

Determining the adsorption energies of small molecules with the intrinsic properties of adsorbates and substrates

Adsorption energies, distances and bader charge analysis of (100) surface.

Nitrogen Adsorption, Dissociation, and Subsurface Diffusion on the Vanadium (110) Surface: A DFT Study for the Nitrogen-Selective Catalytic Membrane Application

Adsorption configurations of NO, NO2 and N2O onto intrinsic graphene

An Insight into Electrostatic Field Effects on SO3 Adsorption by CaO with CO2, SO2 and H2O: A DFT Approach - Aerosol and Air Quality Research

Determining the adsorption energies of small molecules with the intrinsic properties of adsorbates and substrates

Charge density difference upon the adsorption of water on the