Philip Morris International meets with FDA to make its case for
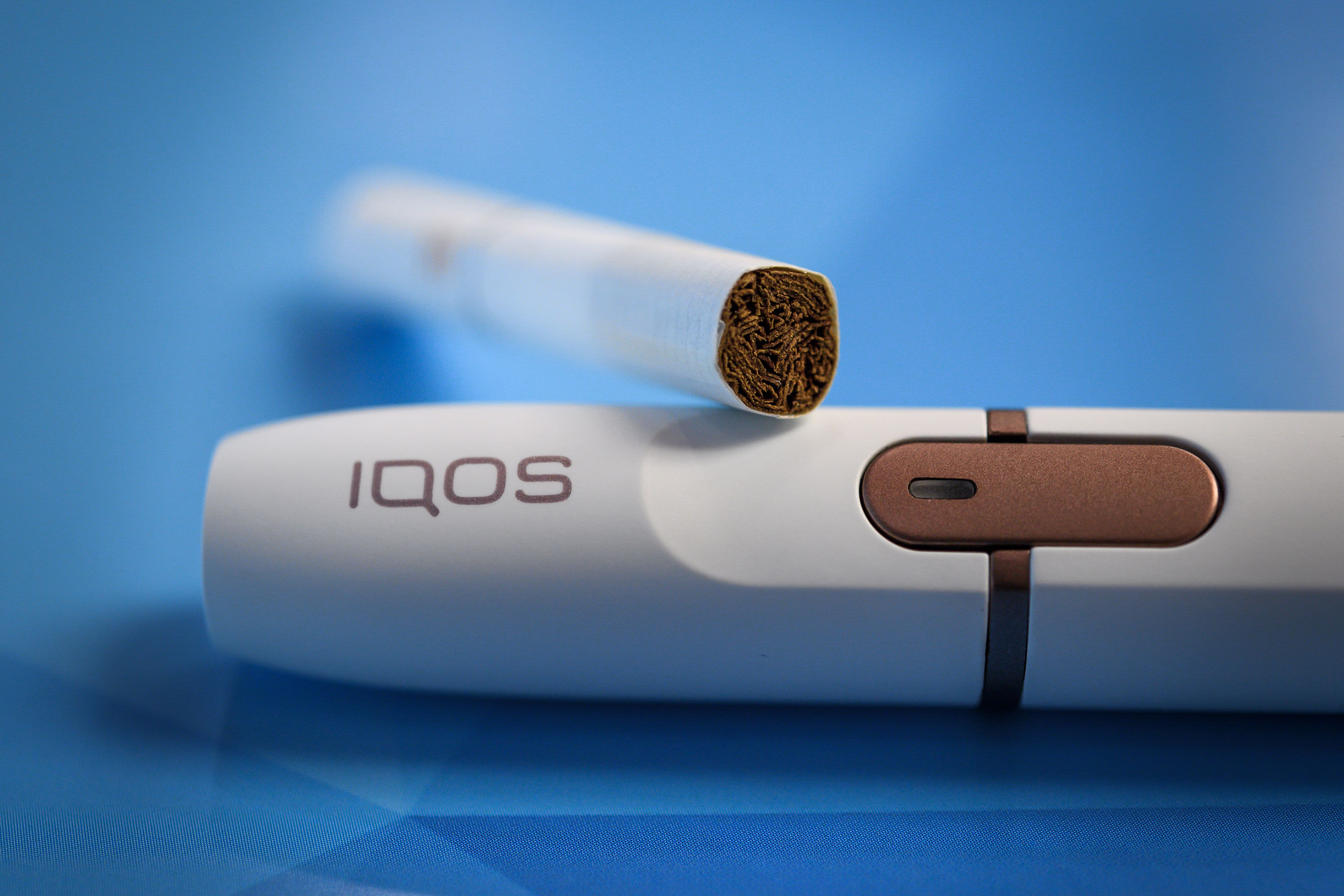

IQOS to be marketed as Modified Risk Tobacco Product after FDA
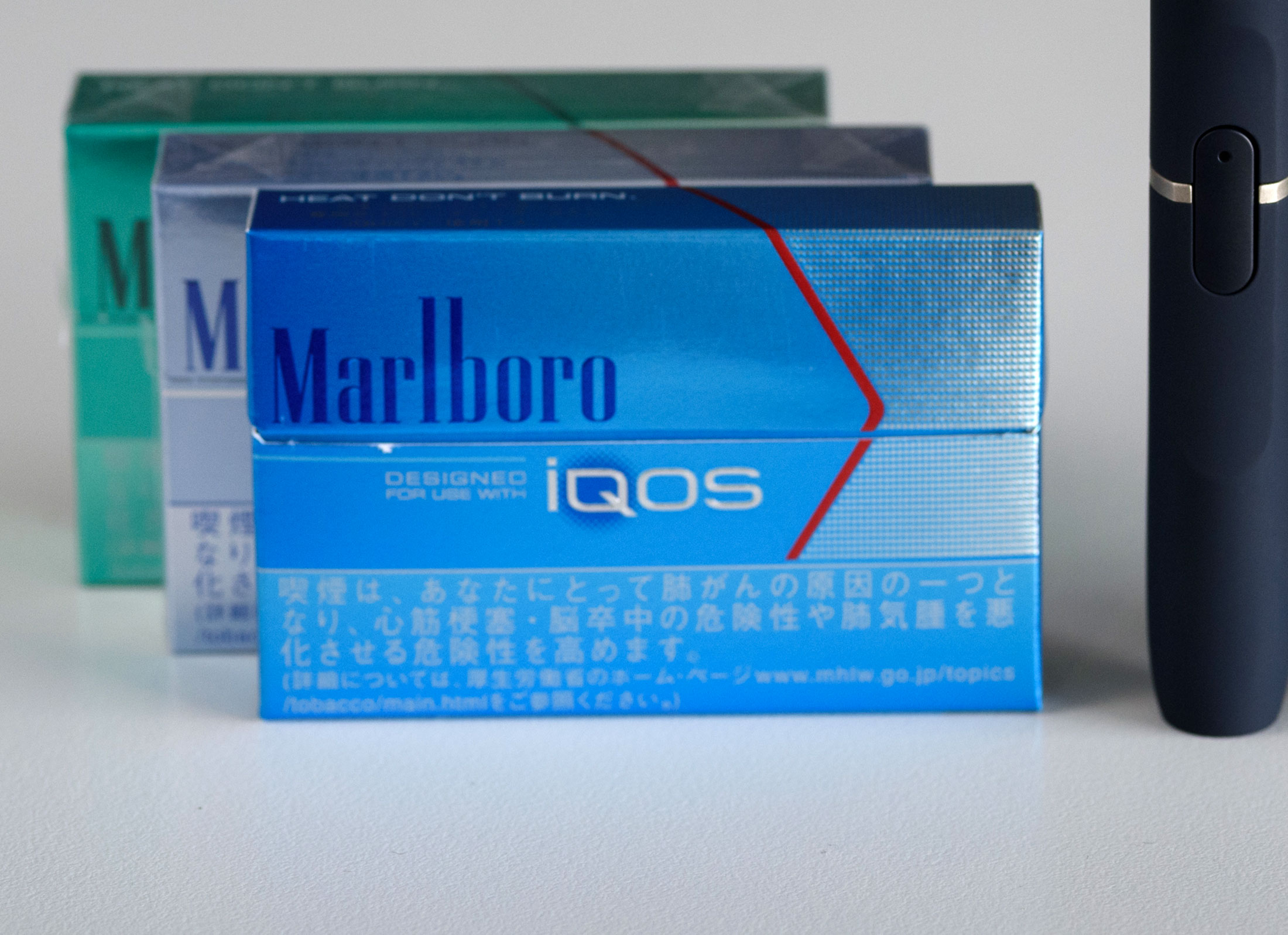
Philip Morris's Cigarette Alternative Could Hit U.S. in 2017

Why we want to stop cigarette sale —Safakish, Philip Morris MD

Startup seeks approval for inhaler that would help smokers quit

Philip Morris Stock: New Deal, New Debt But Good Place To Hide

Philip Morris gets its ash kicked in Uruguay. Where will it next

Technovation 2022 - Diplomacy&Commerce

Philip Morris International CEO Jacek Olczak on a smoke-free future

Philip Morris International 2017 Annual Report
PMI - Case Study
Reduced exposure message and an example of marketing materials

PMI Hosts 'Technovation' Event – Tobacco Reporter

10 facts about the FDA's modified risk tobacco product

Scientific Update Magazine

iQOS meeting big moment for Philip Morris International and Altria








