FDA Authorizes Sale of the IQOS 3 Tobacco Heating System Device in the United States

Altria Group, Inc. (Altria) (NYSE: MO) announces today that the U.S. Food and Drug Administration (FDA) authorized commercialization of the next gener

fda Archives – Total Product Expo

Heated Tobacco Products: Philip Morris International - TobaccoTactics

FDA authorizes sale of Altria's IQOS 3 device - Virginia Business

FDA Authorizes Several NJOY Products – Tobacco Reporter

Content analysis of IQOS direct mail and email marketing in the US - ScienceDirect

10 facts about the FDA's modified risk tobacco product authorization of IQOS

Heated Tobacco Products - TobaccoTactics

FDA authorizes sale of Altria's IQOS 3 device - Virginia Business

Altria starts first U.S. sales of iQOS heat not burn product in Atlanta market
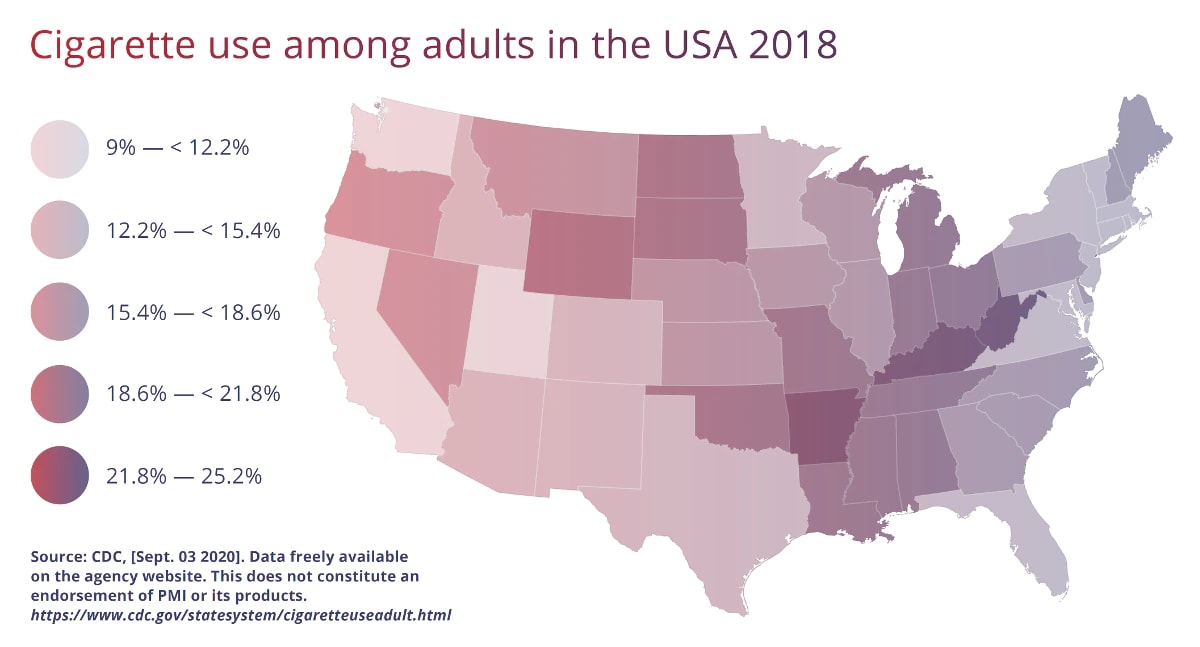
Historic decision made as IQOS Tobacco Heating System is authorized under U.S. MRTP pathway
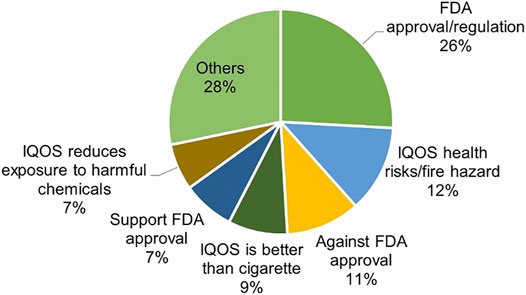
Frontiers Perceptions of the IQOS Heated Tobacco Product on Twitter in the United States

Heated Tobacco Products at the Point of Sale – Counter Tobacco









